
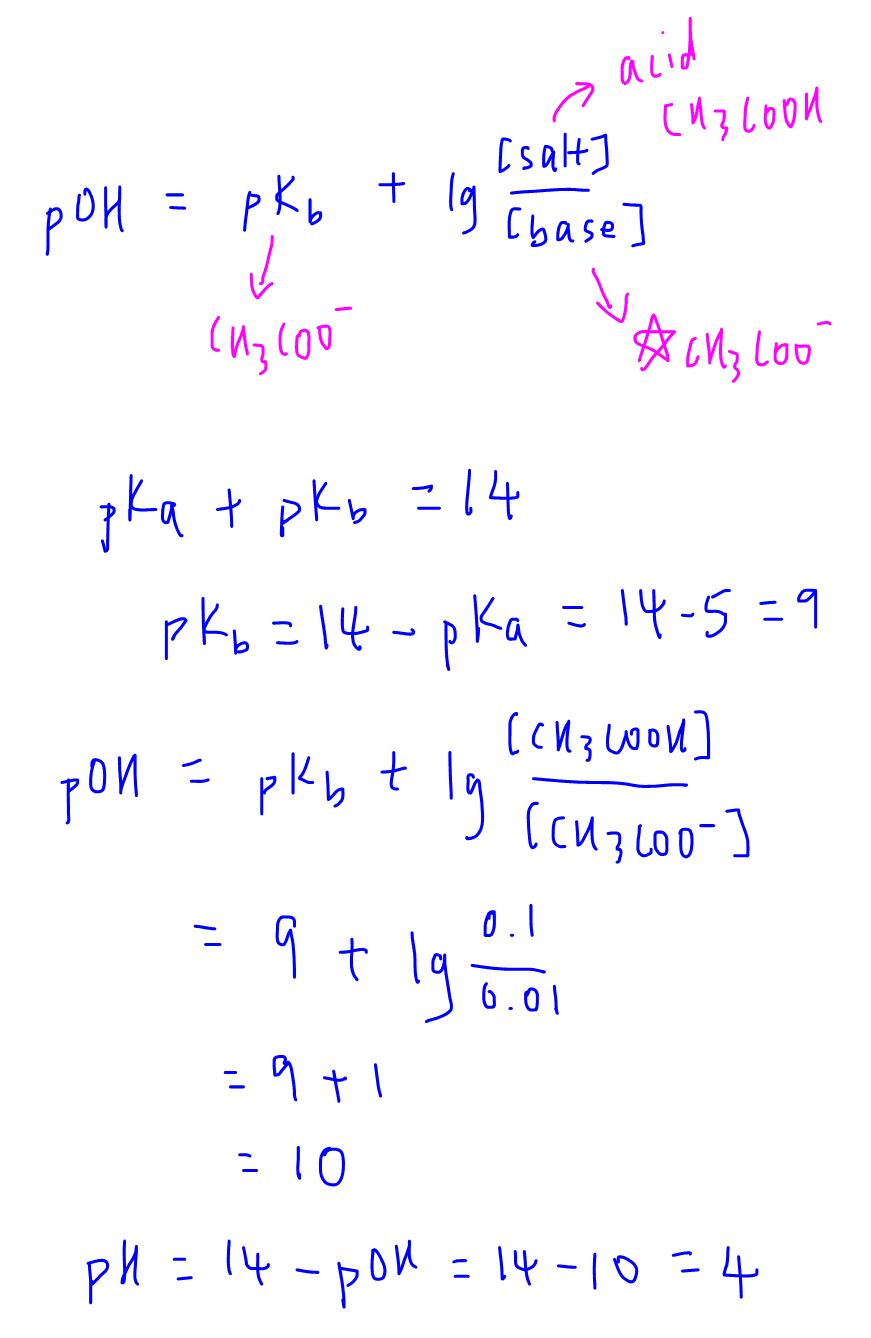
- #SOLUTION CHEMISTRY CALCULATOR HOW TO#
- #SOLUTION CHEMISTRY CALCULATOR SERIAL#
- #SOLUTION CHEMISTRY CALCULATOR SERIES#
In simple displacement reactions, one part of a compounds gets replace by the other part such as followsįor predicting the product of a reaction, the equation balancer is available on the internet. In the synthesis reactions, the final product is the combination of reactants involved in the chemical reaction such as followsĪlso learn what is synthesis in chemistry to understand synthesis completely. It happens due to neutralization reaction such as The chemical reaction between acid and base results in the formation of salt and water. The example is the formation of NaCl such as The chemical reaction between Metal and halogens results in the formation of salt. Related: Complete demonstration of metal displacement reaction in an aqueous medium & structure of DNA.
#SOLUTION CHEMISTRY CALCULATOR HOW TO#
As six tubes are used, the final dilution for the bacteria/cells will be 10 -6 (1 in 1,000,000).Related: How To Write Net Ionic Equations Step by Step? How to predict the product of the following reaction?įor predicting the nature of substance during a chemical reaction, we need to look at to the nature of reacting species and the chemical reaction types. The same process is then repeated for the remaining tube, taking 1 ml from the previous tube and adding it to the next 9 ml diluents. The second tube now has a total dilution factor of 10 -2. Now, 1 ml of mixture is taken from the 10 -1 dilution and is emptied into the second tube. The pipette tip is discarded, and a new pipette tip is attached to the pipette. The dilution is thoroughly mixed by emptying and filling the pipette several times. This provides an initial dilution of 10 -1. The sample is then added to the first tube to make the total volume of 10 ml. 1 ml of properly mixed sample/culture is drawn into the pipette. The sample/culture is taken in a test tube and six test tubes, each with 9 ml of sterile diluents, which can either be distilled water or 0.9% saline, are taken. The following is the procedure for a ten-fold dilution of a sample to a dilution factor of 10 -6: Total dilution factor = previous dilution × dilution of next tube Total dilution factor for the second tube = dilution of first tube × dilution of the second tube.įor the first tube, dilution factor = 10 -1 (1 ml added to 9 ml)įor the second tube, dilution factor = 10 -1 (1ml added to 9 ml). After the first tube, each tube is the dilution of the previous dilution tube. 
In this case, the dilution factor for that test tube will be: The dilution factor of each tube in a set:įor a ten-fold dilution, 1 ml of sample is added to 9 ml of diluent.
#SOLUTION CHEMISTRY CALCULATOR SERIAL#
The dilution factor in a serial dilution can be determined either for an individual test tube or can be calculated as a total dilution factor in the entire series. 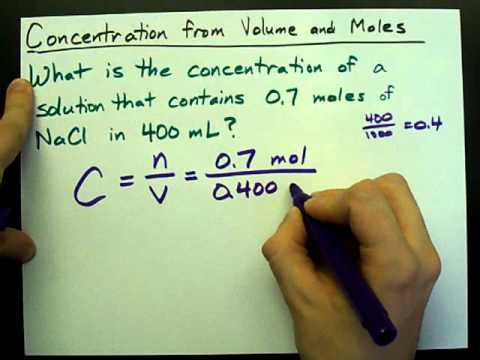
Serial two-fold and ten-fold dilutions are commonly used to titer antibodies or prepare diluted analytes in the laboratory.

In contrast, for a less contaminated sample, a low dilution factor might be sufficient. For e.g., if a water sample is taken from an extremely polluted environment, the dilution factor is increased.
Depending on the estimated concentration of cells/organisms in a sample, the extent of dilution is determined. #SOLUTION CHEMISTRY CALCULATOR SERIES#
Then, a small measured volume of each dilution is used to make a series of pour or spread plates.Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 % saline.


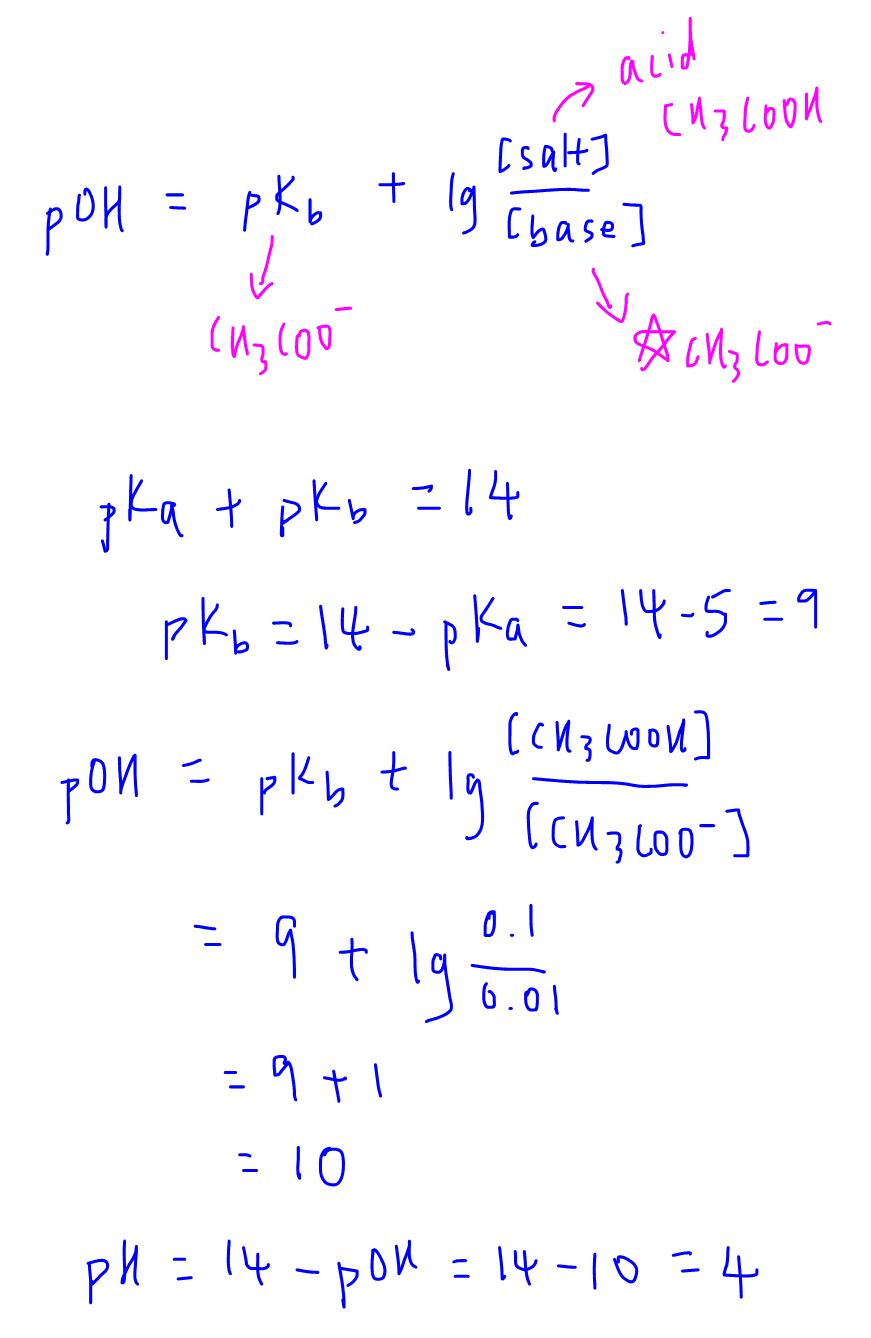





 0 kommentar(er)
0 kommentar(er)
